| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:24:14 UTC |
|---|
| Update Date | 2020-03-13 17:35:09 UTC |
|---|
| BMDB ID | BMDB0062267 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Valyl-Arginine |
|---|
| Description | Valyl-arginine, also known as V-R dipeptide or val-arg, belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. Valyl-arginine is possibly soluble (in water) and a very strong basic compound (based on its pKa). |
|---|
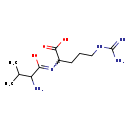
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[(2-Amino-1-hydroxy-3-methylbutylidene)amino]-5-carbamimidamidopentanoate | Generator | | L-Valyl-L-arginine | HMDB | | V-R Dipeptide | HMDB | | Val-arg | HMDB | | Valine arginine dipeptide | HMDB | | Valine-arginine dipeptide | HMDB | | Valylarginine | HMDB | | VR Dipeptide | HMDB |
|
|---|
| Chemical Formula | C11H23N5O3 |
|---|
| Average Molecular Weight | 273.332 |
|---|
| Monoisotopic Molecular Weight | 273.180089627 |
|---|
| IUPAC Name | 2-[(2-amino-1-hydroxy-3-methylbutylidene)amino]-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | 2-[(2-amino-1-hydroxy-3-methylbutylidene)amino]-5-carbamimidamidopentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(C)C(N)C(O)=NC(CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H23N5O3/c1-6(2)8(12)9(17)16-7(10(18)19)4-3-5-15-11(13)14/h6-8H,3-5,12H2,1-2H3,(H,16,17)(H,18,19)(H4,13,14,15) |
|---|
| InChI Key | IBIDRSSEHFLGSD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Valine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Branched fatty acid
- Fatty acyl
- Fatty acid
- Fatty amide
- N-acyl-amine
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Guanidine
- Secondary carboxylic acid amide
- Carboxylic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboximidamide
- Primary aliphatic amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Amine
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dl-9210000000-3ddc6a0fcbbf504d7b03 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-9221000000-fbc3129da319d74b8051 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-5290000000-8c695854fb982cbe0615 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05i0-9310000000-ecd74704281bd02bd6b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9100000000-c66ca6d58e204afbf7d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00e9-1190000000-7bb9b4a772abf68cc478 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053r-7790000000-f71237a9325937d75ef9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9100000000-c1ab72ac083a3598c0c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0490000000-a586ac8c63e42feb9d6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9410000000-9817e3b03422c888644d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0c00-9500000000-dd3f0627ce885307095b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0290000000-1616e2eab7992c6d0461 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05i0-2920000000-695635797408b659812e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-a935f3515b9a1b59447e | View in MoNA |
|---|
|
|---|