| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:25:53 UTC |
|---|
| Update Date | 2020-04-22 15:51:55 UTC |
|---|
| BMDB ID | BMDB0062286 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Hydroxy-3-(4-methoxyphenyl)propanoic acid |
|---|
| Description | 2-Hydroxy-3-(4-methoxyphenyl)propanoic acid belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. Based on a literature review very few articles have been published on 2-Hydroxy-3-(4-methoxyphenyl)propanoic acid. |
|---|
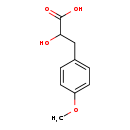
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-3-(4-methoxyphenyl)propanoate | Generator | | 2-Hydroxy-4'-methoxyphloretate | Generator |
|
|---|
| Chemical Formula | C10H12O4 |
|---|
| Average Molecular Weight | 196.1999 |
|---|
| Monoisotopic Molecular Weight | 196.073558872 |
|---|
| IUPAC Name | 2-hydroxy-3-(4-methoxyphenyl)propanoic acid |
|---|
| Traditional Name | 2-hydroxy-3-(4-methoxyphenyl)propanoic acid |
|---|
| CAS Registry Number | 28030-15-1 |
|---|
| SMILES | COC1=CC=C(CC(O)C(O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C10H12O4/c1-14-8-4-2-7(3-5-8)6-9(11)10(12)13/h2-5,9,11H,6H2,1H3,(H,12,13) |
|---|
| InChI Key | GEDLJGYFEOYKEG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenylpropanoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylpropanoic-acid
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Alpha-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Benzenoid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 104 - 105 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fkc-3900000000-89f7497ce29140e69b71 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0100-9362000000-a9b7fc61dc2b5ea348d1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0900000000-6ccbe4588ea44935fe6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ufs-0900000000-b29089901e38719e69d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4900000000-d2af3de1cc46914070c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1900000000-74676802bd7f6840f11f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fdk-1900000000-5e2c775322a1993e89e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-3900000000-cfe6f037808518919f3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9200000000-7dd61f7a544cfbeea2a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9300000000-95b440432758389c7f47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a70-8900000000-152ee7d9babd0a7ff13d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f92-0900000000-5c27ea8c1ae5475e73ef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-3900000000-157ec5bc8dedd58b7f49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bc-9500000000-a0faf64a91f6d7cbdeee | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|