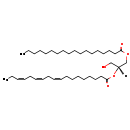
| Description | DG(18:0/18:3(9Z,12Z,15Z)/0:0), also known as diacylglycerol or DAG(18:0/18:3), belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. Thus, DG(18:0/18:3(9Z,12Z,15Z)/0:0) is considered to be a diradylglycerol lipid molecule. DG(18:0/18:3(9Z,12Z,15Z)/0:0) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. DG(18:0/18:3(9Z,12Z,15Z)/0:0) participates in a number of enzymatic reactions, within cattle. In particular, DG(18:0/18:3(9Z,12Z,15Z)/0:0) can be biosynthesized from PA(18:0/18:3(9Z,12Z,15Z)) through its interaction with the enzyme phosphatidate phosphatase. Furthermore, DG(18:0/18:3(9Z,12Z,15Z)/0:0) and eicosadienoyl-CoA can be converted into TG(18:0/18:3(9Z,12Z,15Z)/20:2(11Z,14Z)) through its interaction with the enzyme diacylglycerol O-acyltransferase. Furthermore, DG(18:0/18:3(9Z,12Z,15Z)/0:0) can be biosynthesized from PA(18:0/18:3(9Z,12Z,15Z)); which is mediated by the enzyme phosphatidate phosphatase. Furthermore, DG(18:0/18:3(9Z,12Z,15Z)/0:0) and erucoyl-CoA can be converted into TG(18:0/18:3(9Z,12Z,15Z)/22:1(13Z)) through the action of the enzyme diacylglycerol O-acyltransferase. Furthermore, DG(18:0/18:3(9Z,12Z,15Z)/0:0) can be biosynthesized from PA(18:0/18:3(9Z,12Z,15Z)) through the action of the enzyme phosphatidate phosphatase. Finally, DG(18:0/18:3(9Z,12Z,15Z)/0:0) and docosahexaenoyl-CoA can be converted into TG(18:0/18:3(9Z,12Z,15Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) through its interaction with the enzyme diacylglycerol O-acyltransferase. In cattle, DG(18:0/18:3(9Z,12Z,15Z)/0:0) is involved in a few metabolic pathways, which include de novo triacylglycerol biosynthesis TG(18:0/18:3(9Z,12Z,15Z)/20:2(11Z,14Z)) pathway, de novo triacylglycerol biosynthesis TG(18:0/18:3(9Z,12Z,15Z)/22:1(13Z)) pathway, and de novo triacylglycerol biosynthesis TG(18:0/18:3(9Z,12Z,15Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) pathway. |
|---|
| InChI Identifier | InChI=1S/C39H70O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(41)43-36-37(35-40)44-39(42)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h6,8,12,14,18,20,37,40H,3-5,7,9-11,13,15-17,19,21-36H2,1-2H3/b8-6-,14-12-,20-18-/t37-/m1/s1 |
|---|