| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-04 17:43:26 UTC |
|---|
| Update Date | 2020-04-22 16:09:45 UTC |
|---|
| BMDB ID | BMDB0066106 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | PE(MonoMe(13,5)/MonoMe(13,5)) |
|---|
| Description | Not Available |
|---|
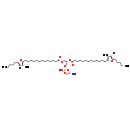
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| PE(MonoMe(13,5)/MonoMe(13,5)) | SMPDB | | Phosphatidylethanolamine(13M5/13M5) | SMPDB | | Phosphatidylethanolamine(MonoMe(13,5)/MonoMe(13,5)) | SMPDB | | PE(13M5/13M5) | SMPDB |
|
|---|
| Chemical Formula | C51H90NO10P |
|---|
| Average Molecular Weight | 908.2348 |
|---|
| Monoisotopic Molecular Weight | 907.630234617 |
|---|
| IUPAC Name | (2-aminoethoxy)[(2R)-2,3-bis({[13-(3-methyl-5-pentylfuran-2-yl)tridecanoyl]oxy})propoxy]phosphinic acid |
|---|
| Traditional Name | 2-aminoethoxy((2R)-2,3-bis({[13-(3-methyl-5-pentylfuran-2-yl)tridecanoyl]oxy})propoxy)phosphinic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]C1=C(CCCCC)OC(CCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCN)OC(=O)CCCCCCCCCCCCC2=C(C)C([H])=C(CCCCC)O2)=C1C |
|---|
| InChI Identifier | InChI=1S/C51H90NO10P/c1-5-7-25-31-45-39-43(3)48(60-45)33-27-21-17-13-9-11-15-19-23-29-35-50(53)57-41-47(42-59-63(55,56)58-38-37-52)62-51(54)36-30-24-20-16-12-10-14-18-22-28-34-49-44(4)40-46(61-49)32-26-8-6-2/h39-40,47H,5-38,41-42,52H2,1-4H3,(H,55,56)/t47-/m1/s1 |
|---|
| InChI Key | XBSWWNAOCKLXBI-QZNUWAOFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | This compound belongs to the class of organic compounds known as phosphatidylethanolamines. These are glycerophosphoetahnolamines in which two fatty acids are bonded to the glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoethanolamines |
|---|
| Direct Parent | Phosphatidylethanolamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphoethanolamine
- Furanoid fatty acid
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Heteroaromatic compound
- Furan
- Carboxylic acid ester
- Amino acid or derivatives
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Carbonyl group
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9002020111-4169fc326a0818b0e5db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9101110110-039d1acabae62299e7d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9002401200-64a31163611dccfb4a68 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-1109030022-23d4c767cebcc1f3821a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fu-6509010010-8ee042a9ed4d95df66b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-9201000000-4238b3b208bfedc663c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000000109-96242732c32fd5639f51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0000000229-d2fa6b6c809a4c1e3c58 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0100010191-82c9d0ee6ac95cac5719 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000119-404a8294355aaea2b726 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-0000130916-090aeebb36e34e73c139 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0000130911-308c6aedaa9ce6aa77a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001000009-9334881eb50a9d361546 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0001000009-9334881eb50a9d361546 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fr-0309030006-de6195f19ff2cb47ac15 | View in MoNA |
|---|
|
|---|