| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-09 21:43:45 UTC |
|---|
| Update Date | 2020-04-22 18:55:00 UTC |
|---|
| BMDB ID | BMDB0095929 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | D-Phenyllactic acid |
|---|
| Description | D-Phenyllactic acid, also known as (R)-3-phenyl-lactate or delta-phenyllactate, belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. D-Phenyllactic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
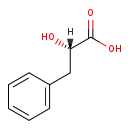
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| ALPHA-HYDROXY-BETA-phenyl-propionIC ACID | ChEBI | | L-(-)-3-Phenyllactic acid | ChEBI | | L-3-Phenyllactic acid | ChEBI | | L-beta-Phenyllactic acid | ChEBI | | a-HYDROXY-b-phenyl-propionate | Generator | | a-HYDROXY-b-phenyl-propionic acid | Generator | | alpha-HYDROXY-beta-phenyl-propionate | Generator | | Α-hydroxy-β-phenyl-propionate | Generator | | Α-hydroxy-β-phenyl-propionic acid | Generator | | L-(-)-3-Phenyllactate | Generator | | L-3-Phenyllactate | Generator | | L-b-Phenyllactate | Generator | | L-b-Phenyllactic acid | Generator | | L-beta-Phenyllactate | Generator | | L-Β-phenyllactate | Generator | | L-Β-phenyllactic acid | Generator | | D-Phenyllactate | Generator | | (+)-2-Hydroxy-3-phenylpropionate | HMDB | | (+)-2-Hydroxy-3-phenylpropionic acid | HMDB | | (+)-3-Phenyllactate | HMDB | | (+)-3-Phenyllactic acid | HMDB | | (+)-b-Phenyllactate | HMDB | | (+)-b-Phenyllactic acid | HMDB | | (+)-beta-Phenyllactate | HMDB | | (+)-beta-Phenyllactic acid | HMDB | | (2R)-2-Hydroxy-2-phenylpropanoate | HMDB | | (2R)-2-Hydroxy-2-phenylpropanoic acid | HMDB | | (2R)-2-Hydroxy-2-phenylpropionate | HMDB | | (2R)-2-Hydroxy-2-phenylpropionic acid | HMDB | | (R)-2-Hydroxy-2-phenylpropionate | HMDB | | (R)-2-Hydroxy-2-phenylpropionic acid | HMDB | | (R)-2-Phenyl-2-hydroxypropanoate | HMDB | | (R)-2-Phenyl-2-hydroxypropanoic acid | HMDB | | (R)-3-Phenyl-lactate | HMDB | | (R)-3-Phenyl-lactic acid | HMDB | | (R)-3-Phenyllactate | HMDB | | (R)-3-Phenyllactic acid | HMDB | | (R)-a-Hydroxy-3-phenylpropionate | HMDB | | (R)-a-Hydroxy-3-phenylpropionic acid | HMDB | | (R)-a-Hydroxy-benzenepropanoate | HMDB | | (R)-a-Hydroxy-benzenepropanoic acid | HMDB | | (R)-alpha-Hydroxy-3-phenylpropionate | HMDB | | (R)-alpha-Hydroxy-3-phenylpropionic acid | HMDB | | (R)-alpha-Hydroxy-benzenepropanoate | HMDB | | (R)-alpha-Hydroxy-benzenepropanoic acid | HMDB | | (R)-b-Phenyllactate | HMDB | | (R)-b-Phenyllactic acid | HMDB | | (R)-beta-Phenyllactate | HMDB | | (R)-beta-Phenyllactic acid | HMDB | | (R)-Phenyllactate | HMDB | | (R)-Phenyllactic acid | HMDB | | b-Phenyl-D-lactate | HMDB | | b-Phenyl-D-lactic acid | HMDB | | beta-Phenyl-delta-lactate | HMDB | | beta-Phenyl-delta-lactic acid | HMDB | | D-2-Hydroxy-3-phenylpropionate | HMDB | | D-2-Hydroxy-3-phenylpropionic acid | HMDB | | D-3-Phenyllactate | HMDB | | D-3-Phenyllactic acid | HMDB | | D-b-Phenyllactate | HMDB | | D-b-Phenyllactic acid | HMDB | | delta-2-Hydroxy-3-phenylpropionate | HMDB | | delta-2-Hydroxy-3-phenylpropionic acid | HMDB | | delta-3-Phenyllactate | HMDB | | delta-3-Phenyllactic acid | HMDB | | delta-beta-Phenyllactate | HMDB | | delta-beta-Phenyllactic acid | HMDB | | delta-Phenyllactate | HMDB | | delta-Phenyllactic acid | HMDB | | (S)-3-Phenyllactate | HMDB |
|
|---|
| Chemical Formula | C9H10O3 |
|---|
| Average Molecular Weight | 166.1739 |
|---|
| Monoisotopic Molecular Weight | 166.062994186 |
|---|
| IUPAC Name | (2S)-2-hydroxy-3-phenylpropanoic acid |
|---|
| Traditional Name | L-3-phenyllactic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](O)(CC1=CC=CC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H10O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8,10H,6H2,(H,11,12)/t8-/m0/s1 |
|---|
| InChI Key | VOXXWSYKYCBWHO-QMMMGPOBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanoic acids. Phenylpropanoic acids are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenylpropanoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylpropanoic-acid
- Alpha-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Benzenoid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9100000000-c22a5f17e7fecfb666f2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9100000000-c22a5f17e7fecfb666f2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9300000000-750e52d0df9e2b90be09 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006x-9540000000-c06e2865c7cf1c780f11 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0gb9-1900000000-cc48c5ff57b37ce10c09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01bd-2900000000-92cd31ed8e8e8e87180f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-5900000000-d1b1e4fdbfd8306c9265 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-750584951bb8866056f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2900000000-1f4cdc94d5bb4ac6489e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01bc-6900000000-779d8af9b71dee9ffe5a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9300000000-bb0139bf3ea8e3c03d20 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-5900000000-eb26ccce038261968168 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9300000000-aa44337c740dacf05e9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-6509f4478fff1766b65a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-3900000000-15a50bcc903d54af3f43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9400000000-0d7b49d15700ae266645 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-ca99933d289067e5347a | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|