| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:41 UTC |
|---|
| Update Date | 2020-04-22 18:55:06 UTC |
|---|
| BMDB ID | BMDB0095948 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Hydroxyestrone-4-S-glutathione |
|---|
| Description | 2-Hydroxyestrone-4-S-glutathione belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review very few articles have been published on 2-Hydroxyestrone-4-S-glutathione. |
|---|
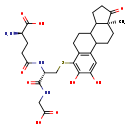
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dihydroxy-1,3,5[10]-estratriene-17-one-4-S-glutathione | HMDB | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-4,5-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-6-yl]sulfanyl}ethyl]-C-hydroxycarbonimidoyl}butanoate | Generator | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-4,5-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-6-yl]sulphanyl}ethyl]-C-hydroxycarbonimidoyl}butanoate | Generator | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-4,5-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-6-yl]sulphanyl}ethyl]-C-hydroxycarbonimidoyl}butanoic acid | Generator |
|
|---|
| Chemical Formula | C28H37N3O9S |
|---|
| Average Molecular Weight | 591.673 |
|---|
| Monoisotopic Molecular Weight | 591.225050487 |
|---|
| IUPAC Name | (2R)-2-amino-4-{[(1S)-1-[(carboxymethyl)carbamoyl]-2-{[(15S)-4,5-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-6-yl]sulfanyl}ethyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | (2R)-2-amino-4-{[(1S)-1-(carboxymethylcarbamoyl)-2-{[(15S)-4,5-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-6-yl]sulfanyl}ethyl]carbamoyl}butanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@]12CCC3C(CCC4=C3C=C(O)C(O)=C4SC[C@@H](NC(=O)CC[C@@H](N)C(O)=O)C(=O)NCC(O)=O)C1CCC2=O |
|---|
| InChI Identifier | InChI=1S/C28H37N3O9S/c1-28-9-8-13-14(17(28)4-6-21(28)33)2-3-15-16(13)10-20(32)24(37)25(15)41-12-19(26(38)30-11-23(35)36)31-22(34)7-5-18(29)27(39)40/h10,13-14,17-19,32,37H,2-9,11-12,29H2,1H3,(H,30,38)(H,31,34)(H,35,36)(H,39,40)/t13?,14?,17?,18-,19-,28+/m1/s1 |
|---|
| InChI Key | ZKSQMGPMDHYFMB-GEAYSQNSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Estrogen-skeleton
- 3-hydroxysteroid
- Oxosteroid
- 17-oxosteroid
- Estrane-skeleton
- 2-hydroxysteroid
- Hydroxysteroid
- Steroid
- Glutamine or derivatives
- Phenanthrene
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- D-alpha-amino acid
- Tetralin
- Alpha-amino acid or derivatives
- Aryl thioether
- Thiophenol ether
- 1-hydroxy-2-unsubstituted benzenoid
- Alkylarylthioether
- N-acyl-amine
- Benzenoid
- Dicarboxylic acid or derivatives
- Fatty acyl
- Fatty amide
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Ketone
- Amino acid
- Thioether
- Sulfenyl compound
- Carboxylic acid
- Primary amine
- Hydrocarbon derivative
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organosulfur compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-1100390000-ea6f60457598e8f42568 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014r-2100049000-ce960d109ca403ad970e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("2-Hydroxyestrone-4-S-glutathione,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fv-1010290000-b13b7a44f4c5a0dc6c03 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07g1-8114890000-3bb454822132467bfbba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-7594210000-219a94916f8b3594d2c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00r6-0024090000-b6e7489aa94b1f7c15bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0049010000-ca91b626a03514e124fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00r5-3933000000-faf6af1383fd365058ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05tf-0035290000-5b6d16b2823e11907e11 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-062a-1450970000-01361acb4f47ee865e7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-8942400000-f7346e6e6a9743a00bf9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0050090000-108be299bab414081576 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-059x-1970030000-8879e7a7bde67b0ca963 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-7910000000-a020a57f2827b9a0bec2 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|