| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:42 UTC |
|---|
| Update Date | 2020-04-22 18:55:07 UTC |
|---|
| BMDB ID | BMDB0095949 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Hydroxyestrone-2-S-glutathione |
|---|
| Description | 4-Hydroxyestrone-2-S-glutathione belongs to the class of organic compounds known as 3-hydroxyacyl coas. These are organic compounds containing a 3-hydroxyl acylated coenzyme A derivative. Based on a literature review very few articles have been published on 4-Hydroxyestrone-2-S-glutathione. |
|---|
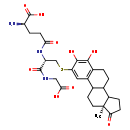
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dihydroxy-1,3,5[10]-estratriene-17-one-2-S-glutathione | HMDB | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-5,6-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-4-yl]sulfanyl}ethyl]-C-hydroxycarbonimidoyl}butanoate | Generator | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-5,6-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-4-yl]sulphanyl}ethyl]-C-hydroxycarbonimidoyl}butanoate | Generator | | (2R)-2-Amino-4-{[(1S)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(15S)-5,6-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2,4,6-trien-4-yl]sulphanyl}ethyl]-C-hydroxycarbonimidoyl}butanoic acid | Generator |
|
|---|
| Chemical Formula | C28H37N3O9S |
|---|
| Average Molecular Weight | 591.673 |
|---|
| Monoisotopic Molecular Weight | 591.225050487 |
|---|
| IUPAC Name | (2R)-2-amino-4-{[(1S)-1-[(carboxymethyl)carbamoyl]-2-{[(15S)-5,6-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-4-yl]sulfanyl}ethyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | (2R)-2-amino-4-{[(1S)-1-(carboxymethylcarbamoyl)-2-{[(15S)-5,6-dihydroxy-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-4-yl]sulfanyl}ethyl]carbamoyl}butanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@]12CCC3C(CCC4=C3C=C(SC[C@@H](NC(=O)CC[C@@H](N)C(O)=O)C(=O)NCC(O)=O)C(O)=C4O)C1CCC2=O |
|---|
| InChI Identifier | InChI=1S/C28H37N3O9S/c1-28-9-8-13-14(17(28)4-6-21(28)32)2-3-15-16(13)10-20(25(37)24(15)36)41-12-19(26(38)30-11-23(34)35)31-22(33)7-5-18(29)27(39)40/h10,13-14,17-19,36-37H,2-9,11-12,29H2,1H3,(H,30,38)(H,31,33)(H,34,35)(H,39,40)/t13?,14?,17?,18-,19-,28+/m1/s1 |
|---|
| InChI Key | UKCFCQKNUGHMIT-GEAYSQNSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-hydroxyacyl coas. These are organic compounds containing a 3-hydroxyl acylated coenzyme A derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | 3-hydroxyacyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Alkyl phosphate
- Imidolactam
- Organic phosphoric acid derivative
- N-substituted imidazole
- Monosaccharide
- Pyrimidine
- Phosphoric acid ester
- Oxolane
- Heteroaromatic compound
- Azole
- Imidazole
- Carbothioic s-ester
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Secondary alcohol
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organoheterocyclic compound
- Azacycle
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Oxacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Primary amine
- Alcohol
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Amine
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-1100390000-5a6ff1a63800b1b2d473 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01bi-5100059000-db27aefd663870cf4215 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("4-Hydroxyestrone-2-S-glutathione,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fv-1010290000-b13b7a44f4c5a0dc6c03 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07g1-8114890000-14ba1b1f0d1ee198833a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-8595210000-dd9ce069c2748eeecbe8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00r6-0024090000-6dd976a53904c370efcd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0049010000-0149d13f0d8a68cb73f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00r5-3933000000-8d3527653652793b9b43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-0050290000-9caf3a62057683843eb5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-5623390000-f40ec3bd5381b83049e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9710000000-42f95767af696508d33c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0011290000-3318a33467aaaa81bea7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0592-2022950000-e22837a99455e890c352 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9516820000-82876537c6b2cc9990c4 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|