| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:46 UTC |
|---|
| Update Date | 2020-04-22 18:55:08 UTC |
|---|
| BMDB ID | BMDB0095953 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7'-Carboxy-alpha-chromanol |
|---|
| Description | 7'-Carboxy-alpha-chromanol belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. Based on a literature review very few articles have been published on 7'-Carboxy-alpha-chromanol. |
|---|
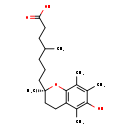
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7-[(2R)-6-Hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylheptanoate | Generator |
|
|---|
| Chemical Formula | C21H32O4 |
|---|
| Average Molecular Weight | 348.4764 |
|---|
| Monoisotopic Molecular Weight | 348.230059512 |
|---|
| IUPAC Name | 7-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylheptanoic acid |
|---|
| Traditional Name | 7-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-1-benzopyran-2-yl]-4-methylheptanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(CCC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2C)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C21H32O4/c1-13(8-9-18(22)23)7-6-11-21(5)12-10-17-16(4)19(24)14(2)15(3)20(17)25-21/h13,24H,6-12H2,1-5H3,(H,22,23)/t13?,21-/m1/s1 |
|---|
| InChI Key | RXYXCDMLSTZSRY-QUXALOBESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-benzopyran
- Medium-chain fatty acid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Oxacycle
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053u-5293000000-80b2982a282ec6f44b33 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-9336600000-1d4eeeeb2edda5e9dfe7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0419000000-31baba58ed21c10befa6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0911000000-fd4b4aaa381aff751e21 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-4900000000-4d99a012ba766d206b62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-f35f40715852d6db1808 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0imj-1419000000-ecba718a31128f597183 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5a-5911000000-8a4a0d088e5e73b598a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ufs-0009000000-e1e2a84ccb56ee11fa93 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-2529000000-835f2f75fc6804660612 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kvx-1980000000-76160bba556a5a5a7be9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ea-0049000000-df4c0025e3d3d977ef64 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-1094000000-6f250936642c49ce490b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-8940000000-8e30675c4791c18df16d | View in MoNA |
|---|
|
|---|