| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:58:47 UTC |
|---|
| Update Date | 2020-04-22 18:55:08 UTC |
|---|
| BMDB ID | BMDB0095954 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7'-Carboxy-alpha-tocotrienol |
|---|
| Description | 7'-Carboxy-alpha-tocotrienol belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. Based on a literature review very few articles have been published on 7'-Carboxy-alpha-tocotrienol. |
|---|
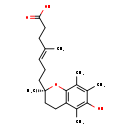
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7'-Carboxy-a-tocotrienol | Generator | | 7'-Carboxy-α-tocotrienol | Generator | | alpha-CMHenHC | HMDB | | (4E)-7-[(2R)-6-Hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylhept-4-enoate | Generator |
|
|---|
| Chemical Formula | C21H30O4 |
|---|
| Average Molecular Weight | 346.4605 |
|---|
| Monoisotopic Molecular Weight | 346.214409448 |
|---|
| IUPAC Name | (4E)-7-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4-methylhept-4-enoic acid |
|---|
| Traditional Name | (4E)-7-[(2R)-6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-1-benzopyran-2-yl]-4-methylhept-4-enoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C\C(CCC(O)=O)=C/CC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2C |
|---|
| InChI Identifier | InChI=1S/C21H30O4/c1-13(8-9-18(22)23)7-6-11-21(5)12-10-17-16(4)19(24)14(2)15(3)20(17)25-21/h7,24H,6,8-12H2,1-5H3,(H,22,23)/b13-7+/t21-/m1/s1 |
|---|
| InChI Key | UZOSSXSASNQFOI-FAKWYAOSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-benzopyran
- Medium-chain fatty acid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Unsaturated fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pj3-5496000000-185d772d98ea7c45a029 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-6425900000-56cb6472446d7a5f54b3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0419000000-d5e7b0fb8d34ed3bdda7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0911000000-7e0202f6433f75d3b3fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-3900000000-8682fadf533be8940884 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-59415d219ec2eecf619b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0imj-1419000000-4596497a27fbfa492f0e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5a-4921000000-c19e057fff6eb5fa62b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0009000000-5b1ae1efb2050db1f95f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-1219000000-edb82ed79fddbdadf977 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fe0-1790000000-ec454dffa7479359bdde | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0098000000-f255d425cdbbf0727346 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05mk-1092000000-32f93aa98cc3606e29d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0api-7950000000-e63cec11840306f7eb18 | View in MoNA |
|---|
|
|---|