| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:00:46 UTC |
|---|
| Update Date | 2020-04-22 18:55:53 UTC |
|---|
| BMDB ID | BMDB0096074 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
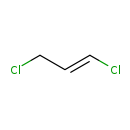
| Common Name | 1,3-Dichloropropene |
|---|
| Description | 1,3-Dichloropropene, also known as D-D 92, belongs to the class of organic compounds known as vinyl chlorides. These are vinyl halides in which a chlorine atom is bonded to an sp2-hybridised carbon atom. 1,3-Dichloropropene exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on 1,3-Dichloropropene. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1E)-1,3-Dichloro-1-propene | ChEBI | | (e)-1,3-Dichloro-1-propene | ChEBI | | D-D 92 | ChEBI | | trans-1,3-Dichloro-1-propene | ChEBI | | trans-1,3-Dichloropropene | ChEBI | | trans-1,3-Dichloropropylene | ChEBI | | trans-3-Chloroallyl chloride | ChEBI | | (1E)-1,3-Dichloroprop-1-ene | HMDB | | (alpha)-Chloroallyl chloride | HMDB | | (alpha,gamma)-Dichloropropylene | HMDB | | (beta)-Epidichlorohydrin cis-trans | HMDB | | (e)-1,3-Dichloropropene | HMDB | | (gamma)-Chloroallyl chloride | HMDB | | 1, 3-Dichloropropene | HMDB | | 1,3-D, Dorlone | HMDB | | 1,3-Dichlopropene | HMDB | | 1,3-dichloro-1-Propene | HMDB, MeSH | | 1,3-dichloro-1-Propene (acd/name 4.0) | HMDB | | 1,3-dichloro-1-Propylene | HMDB | | 1,3-dichloro-2-Propene | HMDB | | 1,3-Dichloroprop-1-ene | HMDB | | 1,3-Dichloropropene (mixed isomers) | HMDB | | 1,3-Dichloropropene (mixed) | HMDB | | 1,3-Dichloropropene (technical grade) | HMDB | | 1,3-DICHLOROPROPENE (telone II) | HMDB | | 1,3-Dichloropropene-1 | HMDB | | 1,3-Dichloropropylene | HMDB, MeSH | | 3-Chloroallyl chloride | HMDB | | 3-Chloropropenyl chloride | HMDB | | 3-Dichloropropylene | HMDB | | alpha,gamma-Dichloropropylene | HMDB | | alpha-Chloroallyl chloride | HMDB | | alpha-Chloroallylchloride | HMDB | | Anema | HMDB | | Chloroallyl chloride | HMDB | | Chloroallylchloride | HMDB | | Chloroorpropenyl chloride | HMDB | | Chloropropenyl chloride | HMDB | | cis-Dichloropropene | HMDB | | D-D Mixture | HMDB | | Dedisol | HMDB | | Di-trapex CP | HMDB | | dichloro-1,3 Propene | HMDB | | Dichloropropene | HMDB | | Dichloropropene, 1,3- (telone II) | HMDB | | Dorlone | HMDB | | Dorlone II | HMDB | | e-1,3-Dichloropropene | HMDB | | gamma-Chloroallyl chloride | HMDB | | gamma-Chloroallylchloride | HMDB | | Sepisol | HMDB | | Sjpdadfhruf`D` | HMDB | | Telone | HMDB | | Telone 2000 | HMDB | | Telone c | HMDB | | Telone C17 | HMDB | | Telone ec | HMDB, MeSH | | Telone II | HMDB, MeSH | | Telone II soil fumigant | HMDB | | Telone II-b | HMDB | | Telone iir | HMDB | | trans-Telone | HMDB | | Tri-form | HMDB | | Vidden D | HMDB | | Vorlex 201 | HMDB | | Zoba eg | HMDB | | 1,3-dichloro-1-Propene, (Z)-isomer | MeSH, HMDB | | 1,3-dichloro-1-Propene, (e)-isomer | MeSH, HMDB | | 1,3-Dichloropropene | MeSH |
|
|---|
| Chemical Formula | C3H4Cl2 |
|---|
| Average Molecular Weight | 110.97 |
|---|
| Monoisotopic Molecular Weight | 109.969005542 |
|---|
| IUPAC Name | (1E)-1,3-dichloroprop-1-ene |
|---|
| Traditional Name | trans-1,3-dichloropropene |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | ClC\C=C\Cl |
|---|
| InChI Identifier | InChI=1S/C3H4Cl2/c4-2-1-3-5/h1-2H,3H2/b2-1+ |
|---|
| InChI Key | UOORRWUZONOOLO-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vinyl chlorides. These are vinyl halides in which a chlorine atom is bonded to an sp2-hybridised carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Vinyl halides |
|---|
| Sub Class | Vinyl chlorides |
|---|
| Direct Parent | Vinyl chlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chloroalkene
- Haloalkene
- Vinyl chloride
- Hydrocarbon derivative
- Organochloride
- Alkyl halide
- Alkyl chloride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004i-9000000000-355c6cbb69f7195b26e7 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004i-9000000000-355c6cbb69f7195b26e7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9200000000-c598c9b2927ffe6d4020 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-f9c91d446ea07605419a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-977e355dc25f96907d7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-9000000000-7fbebdd73428e6aa1542 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1900000000-efde9484329b87cac536 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4900000000-efd53f52cce22e3ba76d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-2d9a0decfdc0fb59dd41 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03k9-5900000000-6420b995013910ad0d39 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03k9-9500000000-1e881a15990580321767 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0229-9000000000-70d2500b930c153a7161 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-d36f52e33fcad27ed733 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-c2fa753da65a4bac80a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-c2fa753da65a4bac80a1 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-004r-9100000000-8ee4722ee13b16f4e04b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CCl4, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|