| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:01:37 UTC |
|---|
| Update Date | 2020-04-22 18:56:13 UTC |
|---|
| BMDB ID | BMDB0096126 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 19-Noraldosterone |
|---|
| Description | 19-Noraldosterone belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Based on a literature review a small amount of articles have been published on 19-Noraldosterone. |
|---|
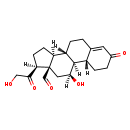
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 19-Noraldosterone | MeSH |
|
|---|
| Chemical Formula | C20H26O5 |
|---|
| Average Molecular Weight | 346.4174 |
|---|
| Monoisotopic Molecular Weight | 346.178023942 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydroxyacetyl)-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-15-carbaldehyde |
|---|
| Traditional Name | (1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydroxyacetyl)-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-15-carbaldehyde |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CCC4=CC(=O)CC[C@]4([H])[C@@]3([H])[C@@]([H])(O)C[C@]12C=O)C(=O)CO |
|---|
| InChI Identifier | InChI=1S/C20H26O5/c21-9-18(25)16-6-5-15-14-3-1-11-7-12(23)2-4-13(11)19(14)17(24)8-20(15,16)10-22/h7,10,13-17,19,21,24H,1-6,8-9H2/t13-,14-,15-,16+,17-,19+,20+/m0/s1 |
|---|
| InChI Key | BIDXSZCVYXAGCG-CRGXURCLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- 20-oxosteroid
- Pregnane-skeleton
- 18-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- 11-beta-hydroxysteroid
- 11-hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic alcohol
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Cyclic ketone
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Primary alcohol
- Aldehyde
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01b9-1928000000-cdb848caaea315dcd467 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-2624900000-c6c50e7558713eca9166 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0019000000-47fe5e2a696f9f14e3a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02dj-0139000000-490647b714129573d2bc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-3292000000-3e3f5f01f4a378e07dee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-be7c4f5948a3f2f2747e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mk-1049000000-3b65522f0f0a481882f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-5092000000-71ea087761c1ccc0cfd1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0049000000-1433d4535573be2c2cd2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0097000000-ade46cff377696999f6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f9m-1392000000-3a2ebdc07dbacfc6c778 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-0059000000-60438fbf0d16d21ce1e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2090000000-e4e3582e2896c4bbf449 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ap3-3092000000-f4f7af746814793ed70a | View in MoNA |
|---|
|
|---|