| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:04 UTC |
|---|
| Update Date | 2020-04-22 18:56:23 UTC |
|---|
| BMDB ID | BMDB0096153 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
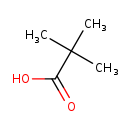
| Common Name | Pivalic acid |
|---|
| Description | Pivalic acid, also known as pivalinsaeure or acide pivalique, belongs to the class of organic compounds known as carboxylic acids. Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. Based on a literature review a significant number of articles have been published on Pivalic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2-Dimethylpropionic acid | ChEBI | | Acide 2,2-dimethylpropanoique | ChEBI | | Acide pivalique | ChEBI | | Acido pivalico | ChEBI | | alpha,alpha-Dimethylpropionic acid | ChEBI | | Dimethylpropionic acid | ChEBI | | Neopentanoic acid | ChEBI | | Pivalinsaeure | ChEBI | | Tert-pentanoic acid | ChEBI | | Trimethylacetic acid | ChEBI | | 2,2-Dimethylpropionate | Generator | | a,a-Dimethylpropionate | Generator | | a,a-Dimethylpropionic acid | Generator | | alpha,alpha-Dimethylpropionate | Generator | | Α,α-dimethylpropionate | Generator | | Α,α-dimethylpropionic acid | Generator | | Dimethylpropionate | Generator | | Neopentanoate | Generator | | Tert-pentanoate | Generator | | Trimethylacetate | Generator | | Pivalate | Generator | | 2,2-Dimethyl-propanoic acid | HMDB | | 2,2-Dimethyl-propionic acid | HMDB | | 2,2-Dimethylpropanoic acid | HMDB | | Kyselina 2,2-dimethylpropionova | HMDB | | Kyselina pivalova | HMDB | | Neovaleric acid | HMDB | | Pivalic acid (acd/name 4.0) | HMDB | | Pivalinsaure | HMDB | | Tert-C4H9COOH | HMDB | | Trimethyl-acetic acid | HMDB | | Versatic 5 | HMDB | | Pivalic acid, sodium salt | HMDB |
|
|---|
| Chemical Formula | C5H10O2 |
|---|
| Average Molecular Weight | 102.1317 |
|---|
| Monoisotopic Molecular Weight | 102.068079564 |
|---|
| IUPAC Name | 2,2-dimethylpropanoic acid |
|---|
| Traditional Name | pivalic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(C)(C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H10O2/c1-5(2,3)4(6)7/h1-3H3,(H,6,7) |
|---|
| InChI Key | IUGYQRQAERSCNH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboxylic acids. Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acids |
|---|
| Direct Parent | Carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-e957b65f703c5d0a437e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0udi-4900000000-230e0140d18957e6c133 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-e957b65f703c5d0a437e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0udi-4900000000-230e0140d18957e6c133 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9100000000-9c9424249e591db177fb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0abl-9100000000-d0ef6bd0ff990f0e982a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-3900000000-2fa44ebd114efa84c1eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-50d93d8a6c80fafb7314 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-f9cb2c03b6c1d9852f83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-22a1ac6f3a1952ac0e52 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-6c14e98fce7c91ecf298 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-9500000000-17d0ccd01dbbf5d59427 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-4c159e60dacc58e6ba98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-4c159e60dacc58e6ba98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9l-9200000000-6005f4b54f4cd522cb5d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-87a9e3b6de2688f21ca9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-9290e064976d791d3ebf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-1398b3c94c7e4a370a6b | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|