| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:17 UTC |
|---|
| Update Date | 2020-04-22 18:56:28 UTC |
|---|
| BMDB ID | BMDB0096166 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
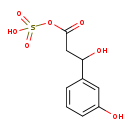
| Common Name | 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulphate |
|---|
| Description | 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulphate belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. Elevated levels of HPHPA have been reported in the urine of children with autism as well as in adult patients with schizophrenia. Recently it has been reported that HPHPA is actually an abnormal phenylalanine metabolite arising from bacterial metabolism in the gastrointestinal tract. 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulphate is an extremely weak basic (essentially neutral) compound (based on its pKa). It has been proposed that HPHPA may be a bacterial metabolite of m-tyrosine, a tyrosine analog that causes symptoms of autism in experimental animals. However, there has been a considerable degree of ambiguity in the origin and/or significance of this compound (PMID:11978597 ). 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulphate is the conjugate of 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid and sulphate. It is thought that the presence of this acid is from nutritional sources (i.e. dietary phenylalanine). Specifically HPHPA appears to arise from the action of the anaerobic bacteria Clostrida sp. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxy-3-(3-hydroxyphenyl)propanoate-O-sulfate | Generator | | 3-Hydroxy-3-(3-hydroxyphenyl)propanoate-O-sulphate | Generator | | 3-Hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulfuric acid | Generator | | 3-Hydroxy-3-(3-hydroxyphenyl)propanoic acid-O-sulphuric acid | Generator | | SulfO 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid | Generator | | SulphO 3-hydroxy-3-(3-hydroxyphenyl)propanoate | Generator | | SulphO 3-hydroxy-3-(3-hydroxyphenyl)propanoic acid | Generator |
|
|---|
| Chemical Formula | C9H10O7S |
|---|
| Average Molecular Weight | 262.237 |
|---|
| Monoisotopic Molecular Weight | 262.014723364 |
|---|
| IUPAC Name | sulfo 3-hydroxy-3-(3-hydroxyphenyl)propanoate |
|---|
| Traditional Name | sulfo 3-hydroxy-3-(3-hydroxyphenyl)propanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(CC(=O)OS(O)(=O)=O)C1=CC(O)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H10O7S/c10-7-3-1-2-6(4-7)8(11)5-9(12)16-17(13,14)15/h1-4,8,10-11H,5H2,(H,13,14,15) |
|---|
| InChI Key | MDWXOTLIYUYWJP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Beta-hydroxy acid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfuric acid monoester
- Organic sulfuric acid or derivatives
- Carboxylic acid salt
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic salt
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-6910000000-6726ada2cb27b9a9ed5e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014j-6389000000-69e59de0ac7859b46e30 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0590000000-7d7d3a0d10ecbf6a581f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2930000000-5de28124a2fa6fdad35c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-4900000000-8d1c25738ded9744f8b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1390000000-52d3ff69936fbe013d09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03du-3920000000-451facc51d79d3fa3f3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001l-9400000000-3f512cd6df10fe81bb78 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03xr-0950000000-97aa03a9cc9409dbd665 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2900000000-9fb66e55bb9f0f4cf937 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mk-9500000000-85ab58495191d2d9cc6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-675e07719da12f0149cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9100000000-ca236561b1d65d853c46 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1900000000-6ac09489e16983d8b680 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|