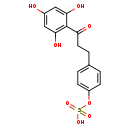| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:43 UTC |
|---|
| Update Date | 2020-04-22 18:56:38 UTC |
|---|
| BMDB ID | BMDB0096193 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Dihydronaringenin-O-sulphate |
|---|
| Description | Dihydronaringenin-O-sulphate, also known as dihydronaringenin-O-sulfuric acid, belongs to the class of organic compounds known as 2'-hydroxy-dihydrochalcones. These are organic compounds containing dihydrochalcone skeleton that carries a hydroxyl group at the 2'-position. Dihydronaringenin-O-sulphate is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dihydronaringenin-O-sulfate | Generator | | Dihydronaringenin-O-sulfuric acid | Generator | | Dihydronaringenin-O-sulphuric acid | Generator | | {4-[3-oxo-3-(2,4,6-trihydroxyphenyl)propyl]phenyl}oxidanesulfonate | Generator | | {4-[3-oxo-3-(2,4,6-trihydroxyphenyl)propyl]phenyl}oxidanesulphonate | Generator | | {4-[3-oxo-3-(2,4,6-trihydroxyphenyl)propyl]phenyl}oxidanesulphonic acid | Generator |
|
|---|
| Chemical Formula | C15H14O8S |
|---|
| Average Molecular Weight | 354.332 |
|---|
| Monoisotopic Molecular Weight | 354.040938114 |
|---|
| IUPAC Name | {4-[3-oxo-3-(2,4,6-trihydroxyphenyl)propyl]phenyl}oxidanesulfonic acid |
|---|
| Traditional Name | {4-[3-oxo-3-(2,4,6-trihydroxyphenyl)propyl]phenyl}oxidanesulfonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC1=CC(O)=C(C(=O)CCC2=CC=C(OS(O)(=O)=O)C=C2)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C15H14O8S/c16-10-7-13(18)15(14(19)8-10)12(17)6-3-9-1-4-11(5-2-9)23-24(20,21)22/h1-2,4-5,7-8,16,18-19H,3,6H2,(H,20,21,22) |
|---|
| InChI Key | IKBKWHISHDVLNO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2'-hydroxy-dihydrochalcones. These are organic compounds containing dihydrochalcone skeleton that carries a hydroxyl group at the 2'-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | 2'-Hydroxy-dihydrochalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2'-hydroxy-dihydrochalcone
- Cinnamylphenol
- Alkyl-phenylketone
- Phenylsulfate
- Butyrophenone
- Acylphloroglucinol derivative
- Arylsulfate
- Phloroglucinol derivative
- Phenylketone
- Benzenetriol
- Phenoxy compound
- Benzoyl
- Aryl ketone
- Aryl alkyl ketone
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Benzenoid
- Vinylogous acid
- Organic sulfuric acid or derivatives
- Ketone
- Polyol
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1931000000-7cfbde85985bacf11d2a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a4i-2333490000-af36a402cde99e4247a5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0209000000-2d253cd3ca91ab9a8a8c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0952000000-2a7b3101afc15f8a2007 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-3930000000-6a717fa9a498992ac249 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0109000000-5b7b683ce96007f5e99c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-1709000000-81a719da40402e4a4853 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-005d-9721000000-318496c81d0e836fb40e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0119000000-105a8fcdd518fc07178a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-0933000000-91c576280ffb07f0ca35 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-3900000000-ad3b580d924d838c452f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0179000000-1465eb111678dd0856e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gyk-0950000000-eb5518d6e5302bf817f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1910000000-c85dfd3ff12838bf6947 | View in MoNA |
|---|
|
|---|