| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:56 UTC |
|---|
| Update Date | 2020-05-11 20:27:48 UTC |
|---|
| BMDB ID | BMDB0096206 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
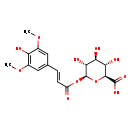
| Common Name | Sinapinic acid-O-glucuronide isomer |
|---|
| Description | Sinapinic acid-O-glucuronide isomer belongs to the class of organic compounds known as hydroxycinnamic acid glycosides. These are glycosylated hydoxycinnamic acids derivatives. Sinapinic acid-O-glucuronide isomer is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sinapinate-O-glucuronide isomer | Generator | | (2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-{[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoyl]oxy}oxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C17H20O11 |
|---|
| Average Molecular Weight | 400.3341 |
|---|
| Monoisotopic Molecular Weight | 400.100561482 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoyl]oxy}oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoyl]oxy}oxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=CC(\C=C\C(=O)O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)=CC(OC)=C1O |
|---|
| InChI Identifier | InChI=1S/C17H20O11/c1-25-8-5-7(6-9(26-2)11(8)19)3-4-10(18)27-17-14(22)12(20)13(21)15(28-17)16(23)24/h3-6,12-15,17,19-22H,1-2H3,(H,23,24)/b4-3+/t12-,13-,14+,15-,17+/m0/s1 |
|---|
| InChI Key | YAIVMBWMHPRYKU-VKAIBYOUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxycinnamic acid glycosides. These are glycosylated hydoxycinnamic acids derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Hydroxycinnamic acid glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxycinnamic acid glycoside
- O-cinnamoyl glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Cinnamic acid ester
- Coumaric acid or derivatives
- Methoxyphenol
- M-dimethoxybenzene
- Dimethoxybenzene
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Styrene
- Alkyl aryl ether
- Fatty acid ester
- Phenol
- Beta-hydroxy acid
- Monosaccharide
- Fatty acyl
- Oxane
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Pyran
- Monocyclic benzene moiety
- Benzenoid
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Secondary alcohol
- Carboxylic acid ester
- Ether
- Acetal
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Polyol
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9234000000-12823e1771d18fe11454 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00di-3141129000-09c8b26505fdc2f1d7a7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0492100000-9fdc4752a8bef6fe8fad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0791000000-4a8e7ec1888f7333443d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-3940000000-699cb959a167d58d6266 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1193000000-409677c5a37121f2b0aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adi-3972000000-9c9c8bf5ea19b610441d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ac0-7960000000-45a3caf04cc9363d3170 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pc0-0249300000-507b919c44e4cde03c92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0951000000-75f62fd02e2f3a87f5c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002r-2963000000-290947eaacd9c7ebbe6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dj-0295000000-eb7abc4704e9c4403f1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3963000000-1ecc7bf05f71c2376328 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08gu-5579000000-75b84d3ca2f5fb88b0bf | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|