| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:24 UTC |
|---|
| Update Date | 2020-04-22 18:56:54 UTC |
|---|
| BMDB ID | BMDB0096234 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
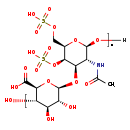
| Common Name | Chondroitin 4,6-disulfate |
|---|
| Description | Chondroitin 4,6-disulfate, also known as chondroitin e sulphate or chondroitinsulfuric acid e, belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. Based on a literature review a small amount of articles have been published on Chondroitin 4,6-disulfate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Chondroitin 4,6-disulfuric acid | Generator | | Chondroitin 4,6-disulphate | Generator | | Chondroitin 4,6-disulphuric acid | Generator | | (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(2R,3R,4R,5R,6R)-2-hydroxy-3-[(1-hydroxyethylidene)amino]-5-(sulfooxy)-6-[(sulfooxy)methyl]oxan-4-yl]oxy}oxane-2-carboxylate | HMDB | | (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(2R,3R,4R,5R,6R)-2-hydroxy-3-[(1-hydroxyethylidene)amino]-5-(sulphooxy)-6-[(sulphooxy)methyl]oxan-4-yl]oxy}oxane-2-carboxylate | HMDB | | (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(2R,3R,4R,5R,6R)-2-hydroxy-3-[(1-hydroxyethylidene)amino]-5-(sulphooxy)-6-[(sulphooxy)methyl]oxan-4-yl]oxy}oxane-2-carboxylic acid | HMDB | | Chondroitin 4,6-sulfate | HMDB | | Chondroitin 4,6-sulphate | HMDB | | Chondroitin e sulfate | HMDB | | Chondroitin e sulphate | HMDB | | Chondroitin sulfate e | HMDB | | Chondroitin sulfate type e | HMDB | | Chondroitin sulphate e | HMDB | | Chondroitin sulphate type e | HMDB | | Chondroitin-4,6-sulfate | HMDB | | Chondroitin-4,6-sulphate | HMDB | | Chondroitinsulfuric acid e | HMDB | | Chondroitinsulfuric acid type e | HMDB | | Chondroitinsulphuric acid e | HMDB | | Chondroitinsulphuric acid type e | HMDB | | Chondroitin 4,6-disulfate | HMDB |
|
|---|
| Chemical Formula | (C14H21NO17S2)nH2O |
|---|
| Average Molecular Weight | Not Available |
|---|
| Monoisotopic Molecular Weight | Not Available |
|---|
| IUPAC Name | Not Available |
|---|
| Traditional Name | Not Available |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]O[C@@H]1O[C@H](COS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H](O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)[C@H]1NC(C)=O |
|---|
| InChI Identifier | InChI=1S/C14H23NO18S2/c1-3(16)15-5-10(31-14-8(19)6(17)7(18)11(32-14)12(20)21)9(33-35(26,27)28)4(30-13(5)22)2-29-34(23,24)25/h4-11,13-14,17-19,22H,2H2,1H3,(H,15,16)(H,20,21)(H,23,24,25)(H,26,27,28)/t4-,5-,6+,7+,8-,9+,10-,11+,13-,14-/m1/s1 |
|---|
| InChI Key | YMRGBHXQASMOMH-MMPMEFKSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- N-acyl-alpha-hexosamine
- Disaccharide sulfate
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Sulfate-ester
- Sulfuric acid monoester
- Pyran
- Sulfuric acid ester
- Oxane
- Alkyl sulfate
- Hydroxy acid
- Acetamide
- Organic sulfuric acid or derivatives
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Hemiacetal
- Organoheterocyclic compound
- Polyol
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Acetal
- Monocarboxylic acid or derivatives
- Alcohol
- Carbonyl group
- Organopnictogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0100090000-9e6691c30df57f7f7e98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0lxt-6701790000-45eb31fcdc09ec18dfd4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000j-9306000000-907157ca3e5ef5a9703b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0001090000-582605201fbeb706b514 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0lzc-3449770000-f8af54efbd658d36dde2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9885320000-e6a613ba1aca81921d3e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|