| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:45:08 UTC |
|---|
| Update Date | 2020-05-05 18:37:44 UTC |
|---|
| BMDB ID | BMDB0001387 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
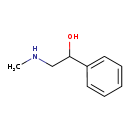
| Common Name | N-Methylphenylethanolamine |
|---|
| Description | N-Methylphenylethanolamine, also known as (+-)-halostachine or MPEOA, belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. N-Methylphenylethanolamine exists in all living organisms, ranging from bacteria to humans. Based on a literature review a small amount of articles have been published on N-Methylphenylethanolamine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-alpha-((Methylamino)methyl)benzenemethanol | ChEBI | | (+-)-Halostachine | ChEBI | | alpha-[(Methylamino)methyl]-benzyl alcohol | Kegg | | (+-)-a-((Methylamino)methyl)benzenemethanol | Generator | | (+-)-Α-((methylamino)methyl)benzenemethanol | Generator | | a-[(Methylamino)methyl]-benzyl alcohol | Generator | | Α-[(methylamino)methyl]-benzyl alcohol | Generator | | MPEOA | MeSH | | N-Methylphenylethanolamine hydrochloride | MeSH | | N-Methylphenylethanolamine hydrochloride, (+-)-isomer | MeSH | | N-Methylphenylethanolamine, (+-)-isomer | MeSH | | N-Methylphenylethanolamine, (R)-isomer | MeSH | | 2-(methylamino)-1-Phenylethanol | HMDB | | 2-methylamino-1-Phenylethanol | HMDB | | alpha-((methylamino)Methyl)-DL-benzyl alcohol | HMDB | | alpha-(Methylaminomethyl)benzyl alcohol | HMDB | | DL-alpha-(Methylaminomethyl)benzyl alcohol | HMDB | | Halostachine | HMDB |
|
|---|
| Chemical Formula | C9H13NO |
|---|
| Average Molecular Weight | 151.2056 |
|---|
| Monoisotopic Molecular Weight | 151.099714043 |
|---|
| IUPAC Name | 2-(methylamino)-1-phenylethan-1-ol |
|---|
| Traditional Name | halostachine |
|---|
| CAS Registry Number | 68579-60-2 |
|---|
| SMILES | CNCC(O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13NO/c1-10-7-9(11)8-5-3-2-4-6-8/h2-6,9-11H,7H2,1H3 |
|---|
| InChI Key | ZCTYHONEGJTYQV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- 1,2-aminoalcohol
- Secondary amine
- Secondary aliphatic amine
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 74 - 76 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0fbl-3900000000-3679c2e020c8e555350d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014i-0900000000-26f7b8d9b9810cdaec56 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0fbl-3900000000-3679c2e020c8e555350d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014i-0900000000-26f7b8d9b9810cdaec56 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-fa15efabc5997da6e3d2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9310000000-f429ae967f272b568609 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0900000000-b348599f4b04bdf562c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0900000000-f90dd7fab94626e11828 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-7900000000-f9602e64157769f32898 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-811f3df9a97a473ef173 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-2900000000-dea7a6b68785d129c556 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9500000000-1250d1db8db14b171869 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ufr-3900000000-0f2b52640015d34b8f97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-8900000000-e361fe87072f77e77c4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-9600000000-cedaced89cfcbb8f5289 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-3900000000-61e18a02be7d3706c2a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9100000000-89cd36302e4945d395eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-bf697c44241fe58f470e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|