| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:39:01 UTC |
|---|
| Update Date | 2020-05-21 16:28:50 UTC |
|---|
| BMDB ID | BMDB0012819 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
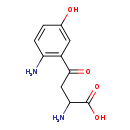
| Common Name | 5-Hydroxykynurenine |
|---|
| Description | 5-Hydroxykynurenine belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. 5-Hydroxykynurenine is possibly soluble (in water) and a very strong basic compound (based on its pKa). 5-Hydroxykynurenine participates in a number of enzymatic reactions, within cattle. In particular, 5-Hydroxykynurenine and formic acid can be biosynthesized from 5-hydroxy-N-formylkynurenine; which is mediated by the enzyme kynurenine formamidase. In addition, 5-Hydroxykynurenine can be converted into 5-hydroxykynurenamine; which is catalyzed by the enzyme aromatic-L-amino-acid decarboxylase. In cattle, 5-hydroxykynurenine is involved in the metabolic pathway called the tryptophan metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-4-(2-amino-5-hydroxyphenyl)-4-oxobutanoic acid | ChEBI | | 2-Amino-4-(2-amino-5-hydroxyphenyl)-4-oxobutanoate | Generator | | 5-Hydroxy-L-kynurenine | HMDB | | 5-Hydroxykynurenine, (L)-isomer | HMDB |
|
|---|
| Chemical Formula | C10H12N2O4 |
|---|
| Average Molecular Weight | 224.2133 |
|---|
| Monoisotopic Molecular Weight | 224.079706882 |
|---|
| IUPAC Name | 2-amino-4-(2-amino-5-hydroxyphenyl)-4-oxobutanoic acid |
|---|
| Traditional Name | 5-hydroxykynurenine |
|---|
| CAS Registry Number | 720-00-3 |
|---|
| SMILES | NC(CC(=O)C1=C(N)C=CC(O)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H12N2O4/c11-7-2-1-5(13)3-6(7)9(14)4-8(12)10(15)16/h1-3,8,13H,4,11-12H2,(H,15,16) |
|---|
| InChI Key | OTDQYOVYQQZAJL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Butyrophenone
- Alpha-amino acid
- Alpha-amino acid or derivatives
- P-aminophenol
- Aminophenol
- Benzoyl
- Gamma-keto acid
- Aryl alkyl ketone
- Aniline or substituted anilines
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Beta-aminoketone
- Keto acid
- Vinylogous amide
- Amino acid
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Organic oxide
- Amine
- Organonitrogen compound
- Primary amine
- Hydrocarbon derivative
- Primary aliphatic amine
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-2900000000-759106525958c7559c0e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9167000000-abb2f436c993df4c1d30 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0890000000-dfa042db46858117582c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03mr-2900000000-c8e51ba13da85857d00b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-5900000000-a8fdd6f4771588adc784 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1090000000-1b6f8b69fc5d1440121f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-9560000000-3a0c724edce767136c71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-6900000000-43fed4337f093b9e8e92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0590000000-3491ab58bac06eaf1c8b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06vi-1900000000-2eca61562922fc2223ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-8900000000-b87794b8cc3c797a29c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05fr-0590000000-5ef5be198ab9accfc633 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-4c1bf27c3b9ad731646d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-9600000000-824917bfb14bccd67e63 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|