| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:39:02 UTC |
|---|
| Update Date | 2020-05-21 16:28:51 UTC |
|---|
| BMDB ID | BMDB0012948 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Formyl-5-hydroxykynurenamine |
|---|
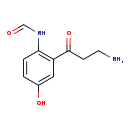
| Description | Formyl-5-hydroxykynurenamine, also known as Formyl-5-hydroxykynurenamine, belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. Formyl-5-hydroxykynurenamine is possibly soluble (in water) and a very strong basic compound (based on its pKa). Formyl-5-hydroxykynurenamine exists in all living organisms, ranging from bacteria to humans. Formyl-5-hydroxykynurenamine can be biosynthesized from serotonin through its interaction with the enzyme indoleamine 2,3-dioxygenase 1. In cattle, formyl-5-hydroxykynurenamine is involved in the metabolic pathway called the tryptophan metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(N-Acetylaminoethyl)-6-hydroxy-5-methoxyindole | ChEBI | | N-(2(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl)acetamide | ChEBI | | N-(2-(6-Hydroxy-5-methoxyindol-3-yl)ethyl)-acetamide | ChEBI | | N-(2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl)-acetamide | HMDB | | N-[2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl]-acetamide | HMDB | | N-[2-(6-Hydroxy-5-methoxyindol-3-yl)ethyl]-acetamide | HMDB |
|
|---|
| Chemical Formula | C10H12N2O3 |
|---|
| Average Molecular Weight | 208.2139 |
|---|
| Monoisotopic Molecular Weight | 208.08479226 |
|---|
| IUPAC Name | N-[2-(3-aminopropanoyl)-4-hydroxyphenyl]formamide |
|---|
| Traditional Name | N-[2-(3-aminopropanoyl)-4-hydroxyphenyl]formamide |
|---|
| CAS Registry Number | 958733-17-0 |
|---|
| SMILES | NCCC(=O)C1=C(NC=O)C=CC(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C10H12N2O3/c11-4-3-10(15)8-5-7(14)1-2-9(8)12-6-13/h1-2,5-6,14H,3-4,11H2,(H,12,13) |
|---|
| InChI Key | CKAXPTWYSHDIBN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Anilide
- Benzoyl
- Aryl alkyl ketone
- N-arylamide
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Beta-aminoketone
- Benzenoid
- Vinylogous amide
- Amino acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-8900000000-a7dfb341fd862129e334 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03di-4920000000-d09c34c768fad3b989b4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06sl-0930000000-9db8e3dae4d6b087e5b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ec-1900000000-218e623c24db925a83c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-3900000000-36941f33d52e98b5147a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-0980000000-ca7dd839f42d8cd4bea9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-6d5837bfc6681842fbb6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054o-6900000000-a30b799e2dc97afbd9b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-2790000000-203573aa857375a3a9a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-8900000000-0583a5d5a5c907fc7967 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-19262f47687e4943cc92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06rx-0930000000-8d93d22f96d424c42e07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08fu-0910000000-9c5660191dd689b05968 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-1900000000-3daac020b14833b9b056 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|