| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:18:28 UTC |
|---|
| Update Date | 2020-05-21 16:28:51 UTC |
|---|
| BMDB ID | BMDB0000015 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Cortexolone |
|---|
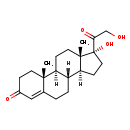
| Description | Cortexolone, also known as cortodoxone or 11 deoxycortisol, belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Thus, cortexolone is considered to be a steroid lipid molecule. Cortexolone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Cortexolone is a potentially toxic compound. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11-Desoxy-17-hydroxycorticosterone | ChEBI | | Cortodoxone | ChEBI | | Reichstein substance S | HMDB | | Substance S, reichstein's | HMDB | | 11 Deoxycortisol | HMDB | | 11 Desoxycortisol | HMDB | | 11 Desoxycortisone | HMDB | | S, Reichstein's substance | HMDB | | 11-Desoxycortisol | HMDB | | 11-Desoxycortisone | HMDB | | Reichsteins substance S | HMDB | | 11-Deoxycortisol | HMDB | | SK&F 3050 | HMDB | | Reichstein's substance S | HMDB | | 11-Deoxy-17-hydroxycorticosterone | HMDB | | 11-Deoxyhydrocortisone | HMDB | | 11-Desoxyhydrocortisone | HMDB | | 17,21-Dihydroxy-4-pregnene-3,20-dione | HMDB | | 17,21-Dihydroxypregn-4-ene-3,20-dione | HMDB | | 17,21-Dihydroxyprogesterone | HMDB | | 17-Hydroxy-11-deoxycorticosterone | HMDB | | 17alpha-Hydroxycortexone | HMDB | | 20-Dione 17,21-dihydroxypregn-4-ene-3 | HMDB | | 4-Pregnene-17alpha,21-diol-3,20-dione | HMDB | | Compound S | HMDB | | 11-Deoxy-17-hydrocorticosterone | HMDB | | 11-Deoxycortisone | HMDB | | 11-Desoxy-17α-hydroxycorticosterone | HMDB | | 11-Desoxy-17alpha-hydroxycorticosterone | HMDB | | 17,21-Dihydroxypregn-4-en-3,20-dione | HMDB | | 17Α,21-dihydroxy-4-pregnen-3,20-dione | HMDB | | 17Α,21-dihydroxypregn-4-ene-3,20-dione | HMDB | | 17Α,21-dihydroxyprogesterone | HMDB | | 17Α-hydroxycortexone | HMDB | | 4-Pregnen-17α,21-diol-3,20-dione | HMDB | | 4-Pregnene-17α,21-diol-3,20-dione | HMDB | | 17alpha,21-Dihydroxy-4-pregnen-3,20-dione | HMDB | | 17alpha,21-Dihydroxypregn-4-ene-3,20-dione | HMDB | | 17alpha,21-Dihydroxyprogesterone | HMDB | | 4-Pregnen-17alpha,21-diol-3,20-dione | HMDB | | Cortexolone | MeSH |
|
|---|
| Chemical Formula | C21H30O4 |
|---|
| Average Molecular Weight | 346.4605 |
|---|
| Monoisotopic Molecular Weight | 346.214409448 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| Traditional Name | (1S,2R,10R,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H30O4/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,25)18(24)12-22/h11,15-17,22,25H,3-10,12H2,1-2H3/t15-,16+,17+,19+,20+,21+/m1/s1 |
|---|
| InChI Key | WHBHBVVOGNECLV-OBQKJFGGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 21-hydroxysteroid
- Progestogin-skeleton
- Pregnane-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Tertiary alcohol
- Cyclic alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - glucocorticoid (CHEBI:28324 )
- deoxycortisol (CHEBI:28324 )
- Glucocorticoids (C05488 )
- C21 steroids (gluco/mineralocorticoids, progestogens) and derivatives (C05488 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030086 )
|
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
- Mitochondria
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 MEOX; 2 TMS) | splash10-0fau-4920000000-b369d2b097d724e4d6d6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0a4j-3931000000-0d5b4b35af4fb7259e0b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4j-3931000000-0d5b4b35af4fb7259e0b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-3a7012051ab6d4bf0562 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-052b-7954000000-8b08129e96f27aa17d97 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-42c6be6599289f724a27 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-052b-6900000000-e4a3639328e2024d0e8f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0009000000-bb32ffe5ad4a80ab7c5a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0390000000-d6fdb36b0e90c40b94cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0029000000-4461b817ffbd52446693 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0cfs-0198000000-95e4ea654010492c731e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01c9-0390000000-2c12ac25b2d03a951073 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-8ffa4f9a93205a223d67 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-3089000000-630c1ceb5066f51ae904 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6091000000-520f7455eb4f28362eb9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-6e67f0e99b733343d1e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08i0-0901000000-78c126f772ed1698f472 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-3930000000-d7d52250ebc31a93ab06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01pa-0049000000-bba8b14791a1510eb866 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-0096000000-364c61e7a9193d6a16fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-0090000000-96af9df83c9954436093 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 50.18 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|