| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:07:19 UTC |
|---|
| Update Date | 2020-05-21 16:28:51 UTC |
|---|
| BMDB ID | BMDB0004030 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 21-Deoxycortisol |
|---|
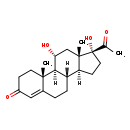
| Description | 21-Deoxycortisol belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. 21-Deoxycortisol is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 21-Deoxycortisol participates in a number of enzymatic reactions, within cattle. In particular, 21-Deoxycortisol can be converted into 11b-hydroxyprogesterone; which is mediated by the enzyme steroid 17-alpha-hydroxylase/17,20 lyase. In addition, 21-Deoxycortisol can be converted into cortisol through its interaction with the enzyme steroid 21-hydroxylase. In cattle, 21-deoxycortisol is involved in the metabolic pathway called the steroidogenesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11b,17-Dihydroxy-pregn-4-ene-3,20-dione | HMDB | | 11b,17-Dihydroxy-progesterone | HMDB | | 11b,17a-Dihydroxypregn-4-ene-3,20-dione | HMDB | | 11b,17a-Dihydroxyprogesterone | HMDB | | 21-Dehydrohydrocortisone | HMDB | | 21-Deoxyhydrocortisone | HMDB | | 21-Desoxycortisol | HMDB | | 4-Pregnene-11beta,17alpha-diol-3,20-dione | HMDB | | Pregn-4-ene-11b,17a-diol-3,20-dione | HMDB |
|
|---|
| Chemical Formula | C21H30O4 |
|---|
| Average Molecular Weight | 346.4605 |
|---|
| Monoisotopic Molecular Weight | 346.214409448 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14R,15S,17R)-14-acetyl-14,17-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | 21-deoxycortisol |
|---|
| CAS Registry Number | 641-77-0 |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)C[C@@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H30O4/c1-12(22)21(25)9-7-16-15-5-4-13-10-14(23)6-8-19(13,2)18(15)17(24)11-20(16,21)3/h10,15-18,24-25H,4-9,11H2,1-3H3/t15-,16-,17+,18+,19-,20-,21-/m0/s1 |
|---|
| InChI Key | LCZBQMKVFQNSJR-CZUPSRJTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Hydroxysteroid
- Oxosteroid
- 11-alpha-hydroxysteroid
- 11-hydroxysteroid
- 17-hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Tertiary alcohol
- Alpha-hydroxy ketone
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Cyclic ketone
- Organic oxide
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05mo-2957000000-d023e738b8e5b9a63cd2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-2116900000-05061eac5c0a64618c6f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0009000000-71aa430e3e20af8b2c95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-0259000000-2fb2818f214612a10bbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4u-2690000000-3dd37cf0a48974795770 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-2d39ebc543dfc8393535 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f9b-0029000000-17d7de8bb31c25cb6a4a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kp0-1096000000-a21e11412ea47ba1ded2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-19287cb485f1fddca890 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-d1621dd0a863681c08e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0079000000-0ce5cfb77bffcbe17979 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0009000000-f6d8370e2ec523e5bab7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1924000000-3c950cee78e9c59f06f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06tf-7960000000-15e137fe577c2459aaf6 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Hill, Martin; Lapcik, Oldrich; Hampl, Richard; Starka, Luboslav; Putz, Zdenek. Radioimmunoassay of three deoxycorticoids in human plasma following HPLC separation. Steroids (1995), 60(9), 615-20. |
|---|